May 14, 2015- By Steven E. Greer, MD
Stroke is the bane of TAVR. In the Edwards Lifesciences Sapien valve, stroke rates exceed 10%. That, along with perivalvular leaks, resulted in death rates of 30% at two-years. Read more »
 June 15, 2014- By Steven E. Greer, MD
June 15, 2014- By Steven E. Greer, MD
34-year-old Erik Compton finished tied for second in the United States Open golf tournament. That was a good accomplishment by any standard, but it was also a genuine feat of modern medicine and surgery because he is living on his third heart. Read more »
March 23, 2014- interviewed by Steven E. Greer, MD
With catheter based methods for replacing the aorta valve not being adopted as expected, the medical device industry is now looking to the mitral valve for hope. Doctors in London and Canada performed Read more »
July 5, 2015- Interviewed by Steven E. Greer, MD
We interviewed William Suh, MD of UCLA about the newly approved Edwards Lifesciences Sapien 3 aortic valve. Read more »
Update April 21, 2015- By Steven E. Greer, MD
As a direct result of our posting of the letter, below, the New York Times then national TV news have picked up this story. The bad press forced Dr. Oz to make a statement on his TV show that he “Will not be Read more »
March 23, 2015- Interviewed by Steven E. Greer, MD
We interviewed the Principal Investigator of the PROMISE trial, Pamela Douglas, MD from Duke. Read more »
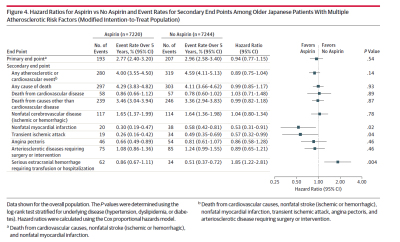
Update January 13, 2015- Yet another study was published highlighting the risks of daily aspirin. Read more »
January 18, 2015- By Steven E. Greer, MD
On July 20th, 2011, the FDA convened an advisory committee to help it determine whether to approve the first ever TAVR device called the Sapien, made by Edwards Lifesciences. I was a public speaker raising the Read more »
 Cardiac surgery, Cardiology/interventional, CMS Medicare Medicaid, Columbia University, FDA, JAMA, Medical Devices, Neurology, Op-Ed, Policy, Univ Pennsylvania | apples49 |
Cardiac surgery, Cardiology/interventional, CMS Medicare Medicaid, Columbia University, FDA, JAMA, Medical Devices, Neurology, Op-Ed, Policy, Univ Pennsylvania | apples49 |  January 25, 2015 6:58 pm |
January 25, 2015 6:58 pm |  Comments (0)
Comments (0)
(best viewed in full-screen)
Update: June 11, 2012
The FDA briefing documents for the June 13 advisory committee to review the PARTNER-A label expansion indicate that the trial was biased by the fact that 7% of the patients randomized to open heart surgery never received surgery. Read more »




